Dipolar recoupling in MAS NMR: A probe for segmental order
in lipid bilayers
KEYWORDS: solid state NMR, magic-angle spinning, 13C-1H
NMR, 2D, structure, dynamics, fluid phase model membranes, dipolar couplings,
J-couplings, magnitude and sign of order parameters, Dipolar Recoupling
On-axis with Scaling and Shape preservation (DROSS), natural abundance
dimyristoylphosphatidylcholine
John D. Gross, Dror E. Warschawski, Robert G. Griffin
ABSTRACT: Novel two-dimensional magic-angle spinning (2D MAS) NMR experiments
designed to measure the magnitudes and signs of 13C-1H dipolar interactions
in fluid phase lipid bilayers are presented: MAS is employed throughout
the experiments while dipolar recoupling is achieved (by radio frequency
irradiation) during the evolution period. The pulse sequence has been called
DROSS for Dipolar Recoupling On-axis with Scaling and Shape preservation.
Multiple 13C-1H dipolar couplings are measured for a natural abundance
sample of La phase dimyristoylphosphatidylcholine (DMPC). The magnitudes
of 13C-1H dipolar interactions are determined by fitting numerical simulations
of recoupled powder lineshapes with experimental data while the signs of
these interactions are obtained by monitoring the build-up of antiphase
magnetization by 13C detection. A comparison of the order parameters obtained
by 13C-1H dipolar recoupling with those previously obtained for DMPC by
2H NMR indicates that dipolar recoupling is a viable method for determining
segmental order in fluid phase lipid bilayers without recourse to isotopic
enrichment. Measurement of the signs of 13C-1H dipolar couplings provides
additional structural information that is unavailable through 2H NMR. The
results obtained for DMPC suggest that the accuracy of the dipolar recoupling
experiments presented in this work is competitive with that of previous
techniques which require switched-angle spinning for the measurement of
the magnitudes and signs of 13C-1H dipolar interactions in lipid bilayers.
REFERENCE: J. D. Gross, D. E. Warschawski and R. G. Griffin, Journal
of the American Chemical Society 119:796-802 (1997)
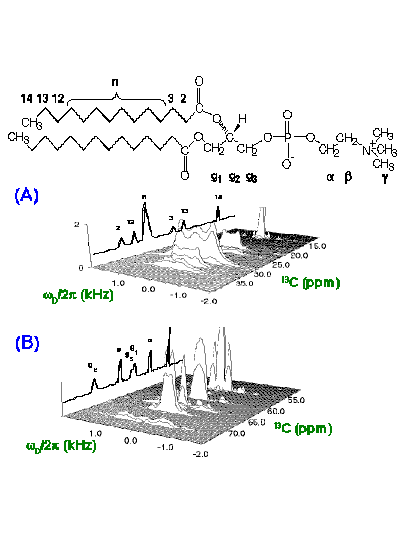
2D 13C-1H DROSS spectrum of natural abundance DMPC at 28C,
spinning at 8kHz. (A) Acyl chain region; (B) Head group and glycerol region.
Membrane peptides: determination of structure and dynamics
under physiological conditions by high-resolution magic-angle spinning NMR
KEYWORDS: solid state NMR, 13C-NMR, 1H-decoupling, magic-angle
spinning, structure, dynamics, fluid phase model membranes, isotopically
labeled peptides, gramicidin A
Dror E. Warschawski, John D. Gross, Robert G. Griffin
ABSTRACT: In the past fifteen years, interferences between molecular
dynamics and coherent manipulation of nuclear magnetization in nuclear
magnetic resonance (NMR) experiments such as spin decoupling, cross-polarization
or magic-angle spinning (MAS) have been identified and studied carefully.
Recent experiments performed in our laboratory on model compounds have
provided insight into the nature of a perturbation responsible for the
broadening of 13C and 15N signals, namely the interference of some molecular
motion with 1H-decoupling. The same effect is demonstrated here for the
first time in the case of a membrane peptide, gramicidin A (gA), in an
hydrated lipid bilayer. Early attempts to obtain high-resolution MAS NMR
spectra of peptides and proteins in liquid-crystalline membranes were unsuccessful
because of problems associated with the dynamics of the molecules. Subsequent
studies have circumvented these issues by performing experiments at very
low temperatures, where motion is quenched. Unfortunately, information
regarding structure and dynamics under physiological conditions is lost.
The present experiment provides the first successful attempt to observe
high-resolution solid-state 13C NMR spectra of a Calpha moiety in membrane
peptide, in an hydrated lipid bilayer in the Lalpha phase. The samples
studied here are synthesized and purified specifically 2H,13C,15N-labeled
gA reconstituted into lipid bilayers. The development of strategies to
circumvent the broadening effect allows us to extract relevant data concerning
the dynamics of gA under physiological conditions. Similar strategies could
be useful for structural and dynamical studies of other membrane peptides
in hydrated bilayers.
REFERENCE: D. E. Warschawski, J. D. Gross and R. G. Griffin, Journal
de Chimie Physique 95:460-466 (1998)

79.9 MHz 13C-MAS-NMR spectra of: a) NCCD2-Gly gA/DMPC/D2O
with 90 kHz 1H-decoupling. T = 25°C, wr = 4.7 kHz, r. d. = 15 s, 2048
scans; b) NCCH2-Gly gA/DMPC/D2O with 90 kHz 1H-decoupling. T = 25°C,
wr = 6.5 kHz, r. d. = 10 s, 2048 scans.

